write electronic configuration of cr|Chromium (Cr) : Baguio In order to write the Na electron configuration we first need to know the .
#BadangisBadang #FuckTheHatersCreditsDIRECTED BY BENZON DANILAASSISTANT DIRECTOR BADANGPRODUCED BY BADANGEDITOR BY BADANGSpecial .
PH0 · What is the electron configuration of chromium? Chemistry Q&A
PH1 · What is the electron configuration of chromium? Chemistry Q&A
PH2 · Electron Configuration of Chromium
PH3 · Electron Configuration for Cr, Cr2+, and Cr3
PH4 · Electron Configuration for Chromium (Cr, Cr2+, Cr3+)
PH5 · Electron Configuration for Chromium (Cr and Cr2+,
PH6 · Electron Configuration Chart of All Elements (Full Chart)
PH7 · Chromium Electron Configuration: 9 (Easy Step
PH8 · Chromium Electron Configuration (Cr) with Orbital Diagram
PH9 · Chromium (Cr)
PH10 · 2.6: Electron Configurations
BitStarz Bonus Codes For Special Promotions. A BitStarz bonus or promo code offers players a unique link to special promotions. They can be available on the BitStarz platform, but you can also find them on third party websites. Selecting a BitStarz free spin code or a Bitstarz sign-up bonus code is a lucrative choice.
write electronic configuration of cr*******In order to write the Chromium electron configuration we first need to know the number of electrons for the Cr atom (there are 24 electrons). Once we have the configuration for Cr, the ions are simple. When we write the configuration we'll put all 24 electrons in .
Both of the configurations have the correct numbers of electrons in each orbital, it is .
write electronic configuration of crBoth of the configurations have the correct numbers of electrons in each orbital, it is .write electronic configuration of cr Chromium (Cr) How to Write the Electron Configuration for Nitrogen (N) Nitrogen is the seventh .
In order to write the Mg electron configuration we first need to know the .When we write the configuration we'll put all 19 electrons in orbitals around the .In order to write the Na electron configuration we first need to know the .Electronic configuration of Cr. Cr is an exception where the last electron enters into the 3d orbital instead of 4s orbital to attain half-filled stability. The electronic configuration .
Mar 23, 2023 To write the configuration for the Chromium ions, first we need to write the electron configuration for just Chromium (Cr). We first need to find the number of electrons for the Cr atom.Chromium (Cr) 24K views 11 years ago Electron Configuration. Electron Configuration of Chromium. Mr. Causey shows you step by step how to write the electron configuration and orbital notation for. chromium 3+ electron configuration. The electronic configuration of Cr 3+ is 1S 2 2S 2 2P 6 3S 2 3P 6 3d 3 4S 0. The number of electrons in the Cr atom is 24, .
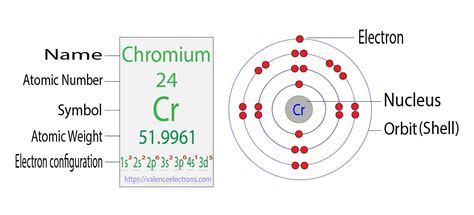
Note: The abbreviated electron configuration of chromium is [Ar] 3d 5 4s 1. When writing an electron configuration, you have to write serially. Chromium ion(Cr 2+, Cr 3+) electron configuration. The ground state .
Chromium is a chemical element with atomic number 24 and represented by the symbol Cr in the Periodic Table. Chromium is a lustrous, hard metal that has a silver-grey colour. It .
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron . In a similar manner, the 3s orbital will have 2 electrons and then 3p will assimilate the 6 further electrons. for the 4s orbital, we will have the 2 electrons and then .
The answer is [Ar] 4s1 3d5Because half-filled subshells are somewhere stable, the atom prefers to have a half-filled 4s and half-filled 3d's than a full 4s a.
Electronic configuration of Chromium, with atomic number 24 = 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 5. Electronic configuration of Copper, with atomic number 29 = 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 10 .
To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: 1s 2. This is the electron configuration of . Filling Transition Metal Orbitals. The electron configuration for the first row transition metals consists of 4s and 3d subshells with an argon (noble gas) core. This only applies to the first row transition metals, adjustments will be necessary when writing the electron configuration for the other rows of transition metals. The noble gas before the .The correct statement (s) about Cr2+ and Mn3+is (are) \lbrack Atomic numbers of Cr 24 and Mn 25\rbrack (A) Cr2\ast is a reducing agent (B) Mn3+ is an oxidizing agent (C) Both Cr2+ and Mn3+ exhibit d4 electronic configuration (D) When Cr2+ is used as a reducing agent, the chromium ion attains d5 electronic configuration. View Solution. The electronic configuration diagram of the Cr atom:. Electronic Configuration Diagram Chromium electron configuration notation. The Cr atom electronic configuration notation is depicted as – [Ar] 4s 1 3d 5.The Cr atom consists of 24 electrons, out of which 18 come under Argon gas configuration, and the remaining three .2,24,283. The electronic configuration of the first 30 elements with atomic numbers listed above corresponds to the ground state of the specific elements. Any configuration that does not correspond to the lowest energy state is called an excited state. To learn more about writing the electronic configuration of an atom or a molecule, visit BYJU . To explain Chromium's electron configuration, we could introduce:. The exchange energy #Pi_e# (a stabilizing quantum mechanical factor that is directly proportional to the number of pairs of electrons in the same subshell or very close-energy subshells with parallel spins); The coulombic repulsion energy #Pi_c# (a destabilizing .Click here:point_up_2:to get an answer to your question :writing_hand:write down the electronic configuration ofi cr3ii pm3iii cu ivThe Aufbau principle predicts that the 4s orbital is always filled before the 3d orbitals, but this is actually not true for most elements!From Sc on, the 3d orbitals are actually lower in energy than the 4s orbital, which means that electrons enter the 3d orbitals first.In this video, we’ll discuss this in more depth and walk through all of the electron configurations for . Thus, the electron configuration of Cr3+ is: Cr3+:1s22s22p63s23p64s03d3. Answer link. Cr^ (3+):1s^ (2)2s^ (2)2p^ (6)3s^ (2)3p^ (6)4s^ (color (red) (0))3d^ (color (red) (3)) The atomic number of Chromium is Z=24, therefore a Cr atom possesses 24 electrons. Cr:1s^ (2)2s^ (2)2p^ (6)3s^ (2)3p^ (6)4s^ (1)3d^ (5) Note .
So the electron configuration for elemental Chromium is. 1s22s22p63s23p64s13d5. And the electrons in the 4s orbital is removed first because this orbital lies further from the nucleus, making electrons easier to remove in ionization. So if we remove 2 electrons to form the Cr2+ ion we remove 1 4s electron and 1 3d electron .

Electron Configuration of Chromium. Mr. Causey shows you step by step how to write the electron configuration and orbital notation for chromium (Cr). Remembe. Cr's electron configuration, following the model would be: \(1s^2 2s^2 2p^6 3s^2 3p^6 4s^23d^4\0, but instead it is \(1s^2 2s^2 2p^6 3s^2 3p^6 4s^13d^5\), because there is extra stability gained from the half-filled d orbital. . Electron configurations are written using the principal quantum number n, followed by the orbital (s, p, d, or f .
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = + 1 2 ).
The Electron: Crash Course Chemistry #5. Video 3.1.2 3.1. 2: An overview of the role of orbitals in electron configurations and how to write electron configurations. The relative energy of the subshells determine the order in which atomic orbitals are filled (1 s, 2 s, 2 p, 3 s, 3 p, 4 s, 3 d, 4 p, and so on). To find the electron configuration of oxygen: Look at the periodic table and find an atomic number of oxygen, which is 8. Fill these 8 electrons in the following order: 1s, 2s, and then 2p. Write the complete electron configuration of oxygen: 1s²2s²2p⁴. Identify the noble gas before oxygen, helium, and write using shorthand notation: [He .Write the probable electronic configuration of chromium and copper. Advertisements. Solution Show Solution. i. The probable (expected) electronic configuration of chromium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 4 4s 2 or [Ar] 3d 4 4s 2.
Book direct at the Comfort Suites Lake Ray Hubbard hotel in Rowlett, TX near The Harbor Rockwall and Downtown Dallas. Free breakfast, free WiFi, outdoor pool.
write electronic configuration of cr|Chromium (Cr)